PFAS FREE PREFILLED SYRINGES
The plungers are manufactured without the coatings commonly related to PFS plungers made from butyl rubber.
These coatings contain PFAS, more specifically contain at least one fully fluorinated methyl (CF3-) or methylene (-CF2-) carbon atom without any H/Cl/Br/I attached to it, which pose a serious challenge to the environment.
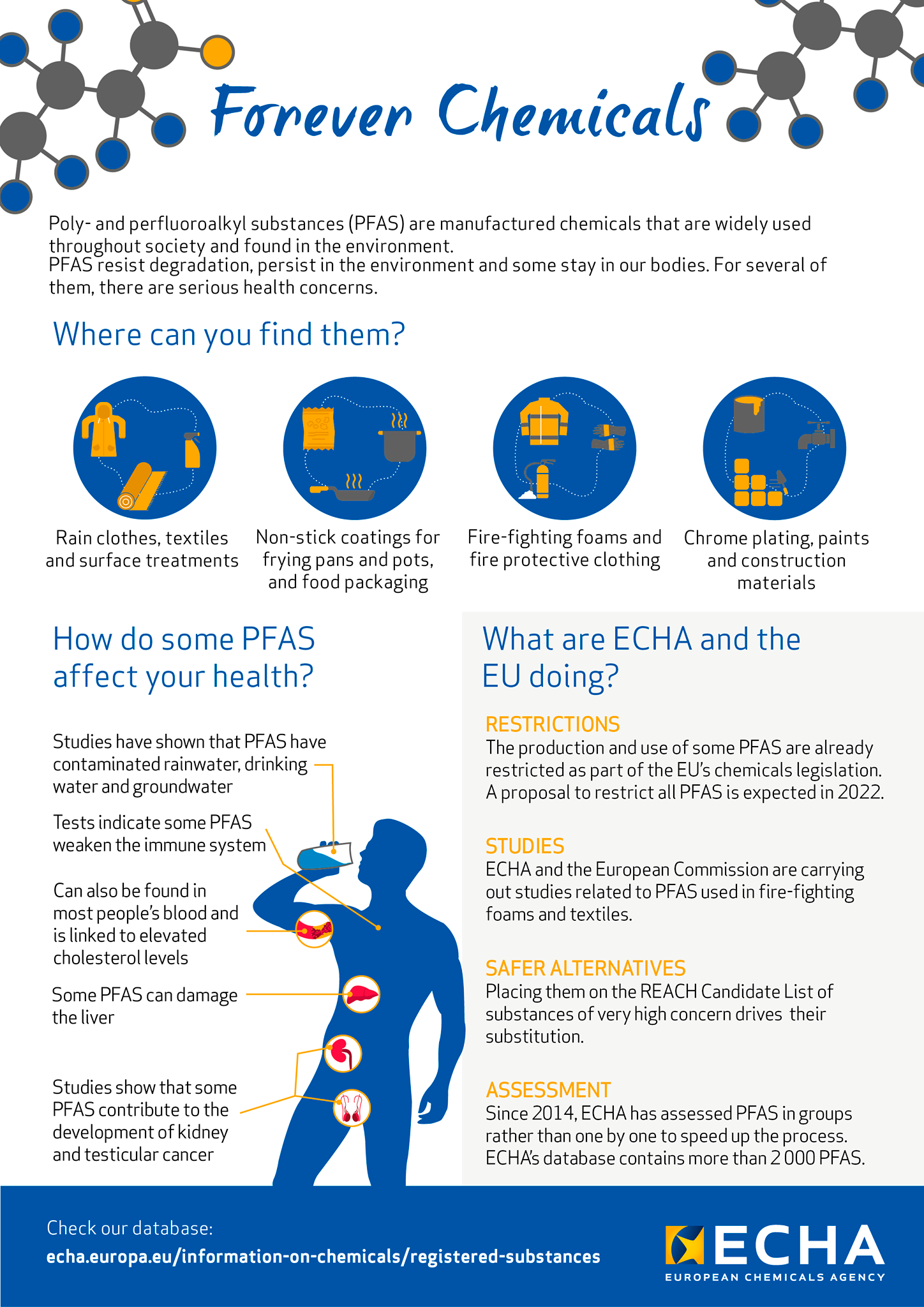
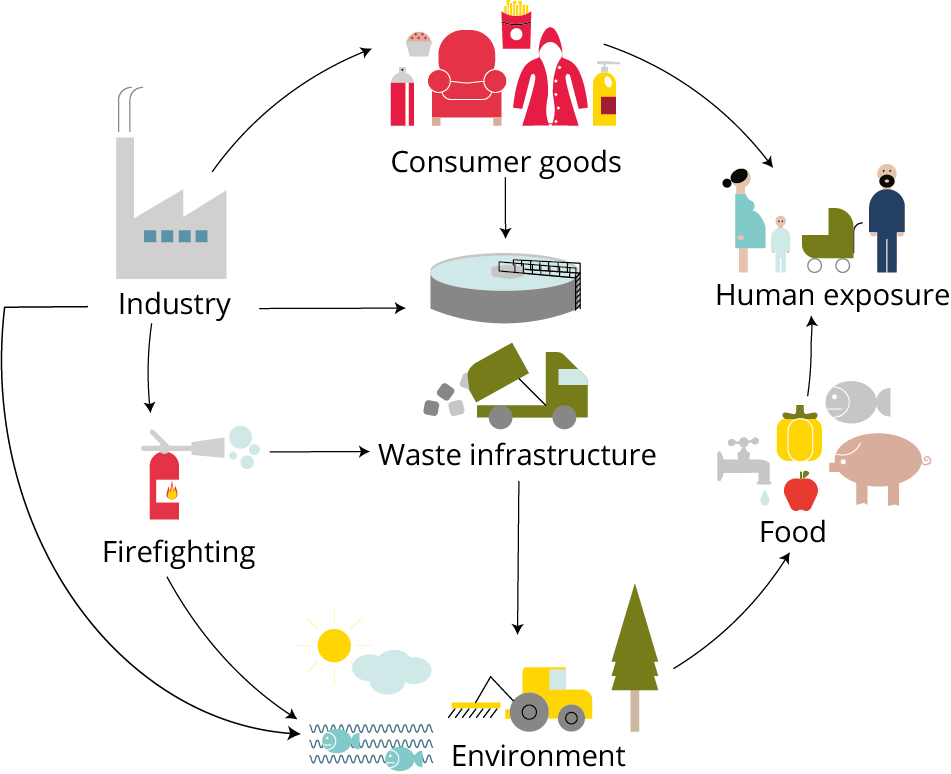
ECHA defines PFAS as “forever chemicals” because PFAS is almost impossible to break down and studies have shown that it has contaminated rainwater, drinking water and groundwater, and when humans are exposed to and consume PFAS it accumulates in the body and can cause lowered immune system, elevated cholesterol levels, liver damage and various kinds of cancer.
The lubrigone material is biocompatible and therefore does not require a coating to protect against chemicals leaching into and interacting with the drug product.
lubrigone contributes to the global environment by offering primary packaging components entirely free of PFAS coatings.
Both our silicone oil free plungers and our auto disabling devices are PFAS free

The EU PFAS restriction proposal
A senior advisor for REACH PFAS restriction responsible for pharma and medical devices expect when the restrictions are implemented, the proposal will either provide a full ban after a 5 or 12 years derogation period (after the transition period) depending of the social economic assessment of the ban .
In short, with the coming change, the majority of the coatings used in parenteral primary packaging are by definition PFAS, which will likely be banned. Consequently, the restrictions will impact the ETFE, PFTE and PCTFE used as coating in primary packaging especially on container closure systems for prefilled syringes, cartridges, and vials, so the business should seek alternatives to the use of such.
When pharmaceutical companies choose the supply format and the primary packaging for a drug product, it is ideally for a long time on the market and therefore selecting and using a primary packaging containing PFAS is a risk that should be considered already now.
Whether the pharmaceutical industry should be concerned about the ban of PFAS already now is a highly relevant question, but moreover the questions should be; what can the pharmaceutical business do to prevent the use and spread of PFAS? Are PFAS coatings in primary packaging really a necessity when there are feasible alternatives? Do we wish to participate in the environmental consequences of the manufacturing and using of PFAS?
On Tuesday 7 February, the five countries presented their proposal for restricting PFASs to European media. This is a recording of their media briefing. ECHA PFAS Proposal
Effects of PFAS on human health
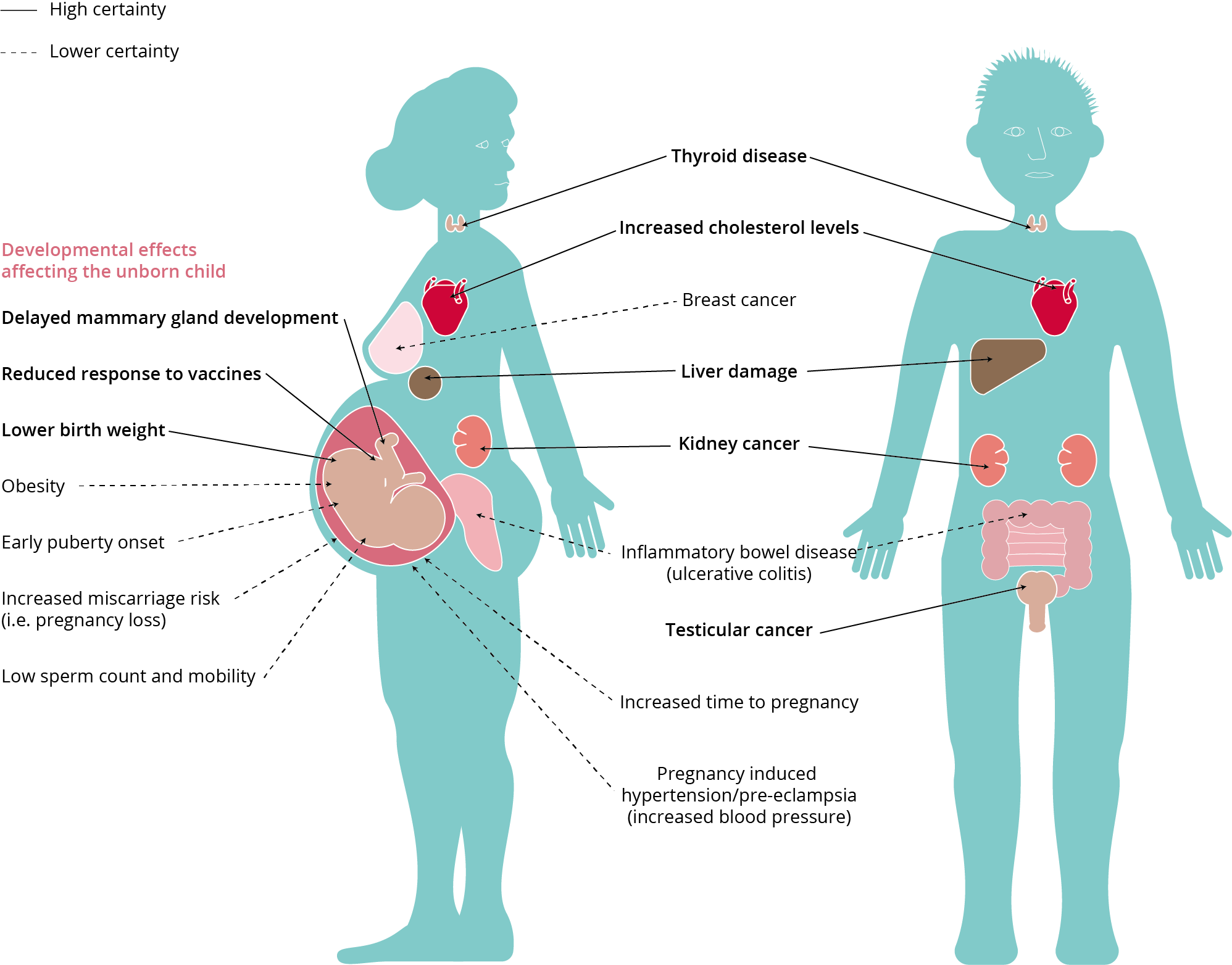
Typical PFAS exposure pathways
- US National Toxicology Program, (2016); C8 Health Project Reports, (2012); WHO IARC, (2017); Barry et al., (2013); Fenton et al., (2009); and White et al., (2011).
- European Environment Agency, Briefing no. 12/2019 – Title: Emerging chemical risks in Europe — ‘PFAS’ – PDF TH-AM-19-014-EN-N – ISBN 978-92-9480-196-8 – ISSN 2467-3196 – doi: 10.2800/486213
HTML TH-AM-19-014-EN-Q – ISBN 978-92-9480-195-1 – ISSN 2467-3196 – doi: 10.2800/02904
Injecto A/S
Pharmaceutical Packaging
Strandvejen 60, 5.
2900 Hellerup
Denmark
Phone: +45 2785 1000
Email: info@injecto.eu
CVR/Org. No.: DK-38 72 98 88
ADVANTAGES
INJECTO can deliver prefillable syringes and syringe components for ready-to-use liquid injectables
Clean injection systems...
Our components are high-precision injection moulded and can be provided for syringe solutions that are lubrication free, coating free, silicone oil free and baked-on silicone free
Feel free to contact INJECTO for more information
 |
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 875949 |


